


We received from Robert Seguin , Managing Partner, of WESTMOUNT CAPITAL , and we are pleased to publish the following note of the analist of Edison Research on the results of Oryzon Genomics on the final analysis from the Phase IIb PORTICO trial.
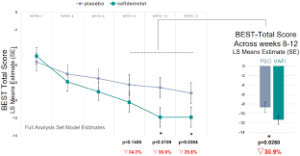
“Oryzon has presented the results of the final analysis from the Phase IIb PORTICO trial, which evaluated the efficacy and safety of vafidemstat in borderline personality disorder (BPD).
While top-line results were initially published in January, the final data (presented at the 37th ECNP 2024 congress) show notable improvements across key efficacy measures, reaffirming the potential of the candidate to deliver meaningful benefits to patients with BPD, a highly underserved condition with no approved drugs.
Management also confirmed that the data, and a registrational Phase III programme, have been discussed with the FDA at an end-of- Phase II (EoP2) meeting. We view this update as highly encouraging for Oryzon and await the announcement of a formal outcome from the FDA on the proposed Phase III programme, which we anticipate in early-Q424″.
care of the redaction
please follow us also on Instagram, website: www.globalmedianews.info
